By Vince Giuliano
This is the third of three blog entries relating to the Nrf2 pathway. The first blog entry The pivotal role of Nrf2. Part 1 – a new view on the control of oxidative damage and generation of hormetic effects dealt with the general mechanisms of operation of Nrf2, and with positive effects of Nrf2 in preventing or treating a number of pathological conditions via its ability to turn on genes for the body’s own antioxidant defense system and genes for combating stress – hundreds of such genes.
The second blog entry The pivotal role of Nrf2. Part 2 – foods, phyto-substances and other substances that turn on Nrf2 deals with how a number of substances that are incidentally antioxidants, plant-derived phyto-substances in particular, actually exercise their benefits through promoting the expression of Nrf2 which in turn activates the body’s own antioxidant and hormetic defense systems. I have added several new items to that blog entry after first publishing it.
This third blog entry explores whether supplementation with substances that promote Nrf2 might be life-extending. Taken together the three blog entries answer a question that has frequently been addressed to me: “How can you simultaneously warn against indiscriminant antioxidant supplementation and at the same time so enthusiastically endorse consuming foods which have strong antioxidant capabilities, ones like broccoli, coffee, olive oil, chocolate, garlic, green tea and blueberries? And, if antioxidants are bad for you, how can you continue to advocate taking so many antioxidant supplements like curcumin, alpha-lipoic acid, ashwagandha, boswellia, ginger and resveratrol?” I explore the question from two viewpoints: first in terms of what the research evidence says, and second from the viewpoint of my personal opinion.
Many researchers see the Nrf2 pathway as being intrinsically involved with aging and longevity.
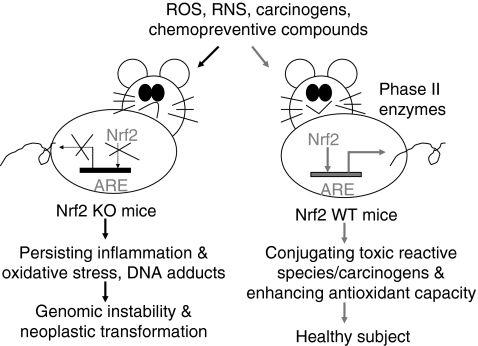
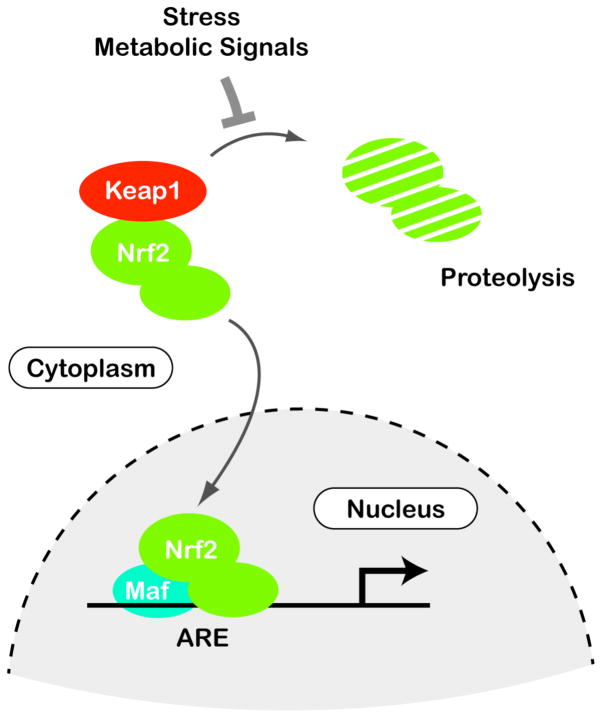
The November 2010 publication Nrf2, a guardian of healthspan and gatekeeper of species longevity states the case for the Nrf2 pathway being critical for longevity. (Italics are mine) “Although aging is a ubiquitous process that prevails in all organisms, the mechanisms governing both the rate of decline in functionality and the age of onset remain elusive. A profound constitutively upregulated cytoprotective response is commonly observed in naturally long-lived species and experimental models of extensions to lifespan (e.g., genetically-altered and/or experimentally manipulated organisms), as indicated by enhanced resistance to stress and upregulated downstream components of the cytoprotective nuclear factor erythroid 2-related factor 2 (Nrf2)-signaling pathway. The transcription factor Nrf2 is constitutively expressed in all tissues, although levels may vary among organs, with the key detoxification organs (kidney and liver) exhibiting highest levels. Nrf2 may be further induced by cellular stressors including endogenous reactive-oxygen species or exogenous electrophiles. The Nrf2-signaling pathway mediates multiple avenues of cytoprotection by activating the transcription of more than 200 genes that are crucial in the metabolism of drugs and toxins, protection against oxidative stress and inflammation, as well as playing an integral role in stability of proteins and in the removal of damaged proteins via proteasomal degradation or autophagy. Nrf2 interacts with other important cell regulators such as tumor suppressor protein 53 (p53) and nuclear factor-kappa beta (NF-κB) and through their combined interactions is the guardian of healthspan, protecting against many age-related diseases including cancer and neurodegeneration. We hypothesize that this signaling pathway plays a critical role in the determination of species longevity and that this pathway may indeed be the master regulator of the aging process.” – “Increased cytoprotection is a key contributor to longevity. We predict that marked differences among species in Nrf2 protein levels and concomitant signaling exist and that they are commensurate with species longevity. In this regard we predict that naturally or genetically-altered long-lived species have more free Nrf2 that is localized in the nucleus, resulting in greater Nrf2 binding to the ARE and thus in an increase in the transcription of cytoprotective target genes such as GST and NQO1, which show greater activity and that this enhanced cellular protection contributes to species longevity. We hypothesize that shorter-lived species, in contrast, show low constitutive levels of Nrf2 with concomitant lower primed defenses against cellular insults and are thus more susceptible to toxins and accumulated damage.
Note that this publication discusses the role of the Nrf2 pathway in terms of species longevity and does not discuss whether deliberate Nrf2 activation might enhance the longevity of members of a specific species such as us humans.
Animal models provide clues as to the role of Nrf2 in controlling longevity. Prime among these is the naked mole rat.
The naked mole rat is a good example: it lives eight times as long as comparable-size mice and has six times the amount of endogenous expression of Nrf2.
I discussed the naked mole rat in the blog entries Animal models of aging – the African naked mole rat and Revisiting the naked mole rat, making the points that naked mole rats have a powerful long-lived antioxidant defense system which mice do not have, and that naked mole rats never get cancers. The November 2011 publication RNA Sequencing Reveals Differential Expression of Mitochondrial and Oxidation Reduction Genes in the Long-Lived Naked Mole-Rat When Compared to Mice reports: “The naked mole-rat (Heterocephalus glaber) is a long-lived, cancer resistant rodent and there is a great interest in identifying the adaptations responsible for these and other of its unique traits. We employed RNA sequencing to compare liver gene expression profiles between naked mole-rats and wild-derived mice. Our results indicate that genes associated with oxidoreduction and mitochondria were expressed at higher relative levels in naked mole-rats. The largest effect is nearly 300-fold higher expression of epithelial cell adhesion molecule (Epcam), a tumour-associated protein. Also of interest are the protease inhibitor, alpha2-macroglobulin (A2m), and the mitochondrial complex II subunit Sdhc, both ageing-related genes found strongly over-expressed in the naked mole-rat. These results hint at possible candidates for specifying species differences in ageing and cancer, and in particular suggest complex alterations in mitochondrial and oxidation reduction pathways in the naked mole-rat.” – “Lastly, one gene recently hypothesized to be important in species longevity is Nrf2 [31], also known as Nfe2l2, which we interestingly found over 6-fold over-expressed in the naked mole-rat (Dataset S1).” My sense is that the higher Nrf2 expression is probably a necessary factor in enabling the naked mole rat’s extraordinary longevity though other characteristics of the naked mole rat’s biochemical systems are also important(ref).
Other long-lived species also appear to have strong antioxidant defense systems in which Nrf2 expression plays a key role, for example turtles(ref)(ref).
The 2008 publication Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila reported: “Keap1/Nrf2 signaling defends organisms against the detrimental effects of oxidative stress and has been suggested to abate its consequences, including aging-associated diseases like neurodegeneration, chronic inflammation, and cancer. Nrf2 is a prominent target for drug discovery, and Nrf2-activating agents are in clinical trials for cancer chemoprevention. However, aberrant activation of Nrf2 by keap1 somatic mutations may contribute to carcinogenesis and promote resistance to chemotherapy. To evaluate potential functions of Keap1 and Nrf2 for organismal homeostasis, we characterized the pathway in Drosophila. We demonstrate that Keap1/Nrf2 signaling in the fruit fly is activated by oxidants, induces antioxidant and detoxification responses, and confers increased tolerance to oxidative stress. Importantly, keap1 loss-of-function mutations extend the lifespan of Drosophila males, supporting a role for Nrf2 signaling in the regulation of longevity. Interestingly, cancer chemopreventive drugs potently stimulate Drosophila Nrf2 activity, suggesting the fruit fly as an experimental system to identify and characterize such agents.”
One view of aging is that it is associated with and partially driven by vascular oxidative stress which is directly linked with Nrf2 expression.
The 2010 publication Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response expounds this view: “There is strong evidence showing that aging is associated with vascular oxidative stress, which has been causally linked to the development of cardiovascular diseases. NF-E2-related factor-2 (Nrf2) is a transcription factor, which is activated by reactive oxygen species in the vasculature of young animals leading to the upregulation of various antioxidant genes. The present study was designed to elucidate age-related changes in the homeostatic role of Nrf2-driven free radical detoxification mechanisms in the vasculature. We found that in the aorta of Fischer 344 × Brown Norway rats, aging results in a progressive increase in O2·− production, and downregulates protein and mRNA expression of Nrf2, which is associated with a decreased nuclear Nrf2 activity and a decrease in the Nrf2 target genes NAD(P)H:quinone oxidoreductase 1, γ-glutamylcysteine synthetase, and heme oxygenase-1. There was an inverse relationship between vascular expression of Nrf2 target genes and age-related increases in the expression of the NF-κB target genes ICAM-1 and IL-6, which was significant by regression analysis. In cultured aorta segments of young (3 mo old) rats treatment with H2O2 and high glucose significantly increases nuclear translocation of Nrf2 and upregulates the expression of Nrf2 target genes. In contrast, in cultured aorta segments of aged (24 mo old) rats, the induction of Nrf2-dependent responses by H2O2 and high glucose are blunted. High glucose-induced vascular oxidative stress was more severe in aortas of aged rats, as shown by the significantly increased H2O2 production in these vessels, compared with responses obtained in aortas from young rats. Moreover, we found that aging progressively increases vascular sensitivity to the proapoptotic effects of H2O2 and high glucose treatments. Taken together, aging is associated with Nrf2 dysfunction in the vasculature, which likely exacerbates age-related cellular oxidative stress and increases sensitivity of aged vessels to oxidative stress-induced cellular damage.” I agree with the authors although aging clearly has to do with a lot more than oxidative vascular stress.
Contrarian research suggests that overexpression of Nrf2 could lead to aortic atherosclerosis, creating more harm than good.
The October 2010 publication NF-E2–Related Factor 2 Promotes Atherosclerosis by Effects on Plasma Lipoproteins and Cholesterol Transport That Overshadow Antioxidant Protectionreports: Objective—To test the hypothesis that NF-E2–related factor 2 (Nrf2) expression plays an antiatherogenic role by its vascular antioxidant and anti-inflammatory properties. Methods and Results—Nrf2 is an important transcription factor that regulates the expression of phase 2 detoxifying enzymes and antioxidant genes. Its expression in vascular cells appears to be an important factor in the protection against vascular oxidative stress and inflammation. We developed Nrf2 heterozygous (HET) and homozygous knockout (KO) mice on an apolipoprotein (apo) E–null background by sequential breeding, resulting in Nrf2−/−, apoE−/− (KO), Nrf2−/+, apoE−/− (HET) and Nrf2+/+, and apoE−/− wild-type littermates. KO mice exhibited decreased levels of antioxidant genes with evidence of increased reactive oxygen species generation compared with wild-type controls. Surprisingly, KO males exhibited 47% and 53% reductions in the degree of aortic atherosclerosis compared with HET or wild-type littermates, respectively. Decreased atherosclerosis in KO mice correlated with lower plasma total cholesterol in a sex-dependent manner. KO mice also had a decreased hepatic cholesterol content and a lower expression of lipogenic genes, suggesting that hepatic lipogenesis could be reduced. In addition, KO mice exhibited atherosclerotic plaques characterized by a lesser macrophage component and decreased foam cell formation in an in vitro lipid-loading assay. This was associated with a lower rate of cholesterol influx, mediated in part by decreased expression of the scavenger receptor CD36. Conclusion—Nrf2 expression unexpectedly promotes atherosclerotic lesion formation in a sex-dependent manner, most likely by a combination of systemic metabolic and local vascular effects.”
According to a Science Daily article on this research: “We were very surprised at the finding,” said principal investigator Dr. Jesus Araujo, director of environmental cardiology at the David Geffen School of Medicine at UCLA. “In fact, the atherosclerosis-producing factors were greater than the antioxidant benefits. The development of novel antioxidant therapies is quite important, and this research may help shed light on why treatments affecting this protein may not be as effective as we thought.” – “For the study, the team was able to isolate and identify Nrf2′s actions by looking at what would happen in mice that were specially bred without the protein. — Researchers found that male mice without Nrf2 had decreased levels of antioxidants, as would be expected, but also exhibited a 53 percent reduction in atherosclerotic plaques in the aorta, compared with normal animals. Mice with only half the gene expression for Nrf2 exhibited the same degree of plaque formation as normal animals.” The research describes reduced atherosclerotic plaque reduction in Nrf2 knockout mice. It does not investigate whether induced overexpression of Nrf2 would lead to additional atherosclerosis although it raises this as a disturbing possibility.
Other contrarian research suggests that quality of antioxidant defense and longevity within a species may not be correlated.
See the blog entries Consistency – “The hobgoblin of small minds?”, Oxidative damage – cause or effect? and The free radical theory of aging. Is it really a theory of aging? “It does not appear to apply to mice living in luxury mouse resorts.”
AMPK expression appears to control the aging process.
The December 2011 e-publication AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network makes the point that control of Nrf2 along with control of other key pathways by AMPK drives aging. “Efficient control of energy metabolic homeostasis, enhanced stress resistance, and qualified cellular housekeeping are the hallmarks of improved healthspan and extended lifespan. AMPK signaling is involved in the regulation of all these characteristics via an integrated signaling network. Many studies with lower organisms have revealed that increased AMPK activity can extend the lifespan. Experiments in mammals have demonstrated that AMPK controls autophagy through mTOR and ULK1 signaling which augment the quality of cellular housekeeping. Moreover, AMPK-induced stimulation of FoxO/DAF-16, Nrf2/SKN-1, and SIRT1 signaling pathways improves cellular stress resistance. Furthermore, inhibition of NF-κB signaling by AMPK suppresses inflammatory responses. Emerging studies indicate that the responsiveness of AMPK signaling clearly declines with aging. The loss of sensitivity of AMPK activation to cellular stress impairs metabolic regulation, increases oxidative stress and reduces autophagic clearance. These age-related changes activate innate immunity defence, triggering a low-grade inflammation and metabolic disorders. We will review in detail the signaling pathways of this integrated network through which AMPK controls energy metabolism, autophagic degradation and stress resistance and ultimately the aging process.”
Also, see the June 2010 blog entry AMPK and longevity.
The July 2011 publication AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan reported: “Adenosine monophosphate-activated protein kinase (AMPK) is a crucial regulator of energy metabolic homeostasis and thus a major survival factor in a variety of metabolic stresses and also in the aging process. Metabolic syndrome is associated with a low-grade, chronic inflammation, primarily in adipose tissue. A low-level of inflammation is also present in the aging process. There are emerging results indicating that AMPK signaling can inhibit the inflammatory responses induced by the nuclear factor-κB (NF-κB) system. The NF-κB subunits are not direct phosphorylation targets of AMPK, but the inhibition of NF-κB signaling is mediated by several downstream targets of AMPK, e.g., SIRT1, PGC-1α, p53, and Forkhead box O (FoxO) factors. AMPK signaling seems to enhance energy metabolism while it can repress inflammatory responses linked to chronic stress, e.g., in nutritional overload and during the aging process. AMPK can inhibit endoplasmic reticulum and oxidative stresses which are involved in metabolic disorders and the aging process. Interestingly, many target proteins of AMPK are so-called longevity factors, e.g., SIRT1, p53, and FoxOs, which not only can increase the stress resistance and extend the lifespan of many organisms but also inhibit the inflammatory responses. The activation capacity of AMPK declines in metabolic stress and with aging which could augment the metabolic diseases and accelerate the aging process. We will review the AMPK pathways involved in the inhibition of NF-κB signaling and suppression of inflammation. We also emphasize that the capacity of AMPK to repress inflammatory responses can have a significant impact on both healthspan and lifespan.”
The 2008 publication NF-kappa B and Nrf2 as potential chemopreventive targets of some anti-inflammatory and antioxidative phytonutrients with anti-inflammatory and antioxidative activities reports: “Chemoprevention refers to the use of defined non-toxic chemical regimens to inhibit, reverse or retard the process of multi-stage carcinogenesis that involves multiple signal transduction events. A new horizon in chemoprevention research is the recent discovery of molecular links between inflammation and cancer. Components of the cell signaling network, especially those that converge on the ubiquitous eukaryotic redox-sensitive transcription factor, nuclear factor-kappa B (NF-kappa B), have been implicated in the pathogenesis of many inflammation-associated disorders. A wide variety of chemopreventive and chemoprotective phytochemicals and phytonutrients can alter or correct undesired cellular functions caused by abnormal pro-inflammatory signal transmissions, mediated by NF-kappaB. Modulation of cellular signaling involved in chronic inflammatory responses, induced by anti-inflammatory agents, hence provides a rational and pragmatic strategy in molecular target-based chemoprevention and cytoprotection. Induction of phase-2 detoxifying or antioxidant genes represents an important cellular defence in response to oxidative and electrophilic insults. Nuclear transcription factor erythroid 2p45 (NF-E2)-related factor 2 (Nrf2) plays a crucial role in regulating phase-2 detoxifying/antioxidant gene induction. Many antioxidants derived from dietary and medicinal plants have been found to activate this particular redox-sensitive transcription factor, thereby potentiating the cellular antioxidant or detoxification capacity.”
The January 2012 publication Phytochemicals suppress nuclear factor-κB signaling: impact on health span and the aging process reports consistently: “Purpose of review: We will briefly review the current knowledge on the major molecular targets of plant-derived phytochemicals, particularly their connections to nuclear factor-κB (NF-κB) signaling, and accordingly link these observations to their anti-inflammatory properties and beneficial effects on the aging process and age-related degenerative diseases. Recent findings: Many of the major phytochemicals, for example, flavonoids and terpenoids, possess significant therapeutic properties including anti-inflammatory and anticancer effects. Although phytochemicals have multiple molecular targets, recent studies have indicated that many of them can activate signaling pathways driven by AMP-activated protein kinase and nuclear factor-erythroid 2-related factor 2. These pathways are potent inhibitors of NF-κB signaling, a crucial inducer of inflammatory responses and cancer formation. Current opinion suggests that inflammation has a critical role in the aging process and in the pathogenesis of age-related degenerative diseases and, thus, anti-inflammatory properties of phytochemicals could explain their beneficial effects on health span and lifespan. Summary: Plant-derived phytochemicals are promising lead compounds helping modern drug discovery to develop potent and safe inhibitors for age-related inflammatory disorders driven by NF-κB signaling.”
WRAPPING IT UP
These four last-mentioned publications make what I believe are very telling points:
- AMPK signaling has positive impacts on both lifespan and healthspan, and this appears to apply across a wide variety of species.
- Multiple phytosubstances are promoters of AMPK and simultaneously are inhibitors of NF-kappaB and are activators of the Nrf2 pathway.
- Health benefits associated with those substancesn are conferred by activation of antioxidant and hormetic response genes by Nrf2, by limitation of inflammation due to inhibition of NF-kappaB expression and by a variety of associated mechanisms.
Another key point is that:
- The redox control pathways in which Nrf2 plays a key role and the NF-kappaB pathways controlling inflammation and driving many disease processes are in close interaction.
From the March 2011 publication From Normal Cells to Malignancy: Distinct Role of Pro-inflammatory Factors and Cellular Redox Mechanisms: “The genesis of many solid cancers is a complex, multistep process that includes cellular neoplastic transformation, resistance to apoptosis, loss of control of cell cycle, angiogenesis and acquisition of invasive properties. Among a number of factors, the simultaneous existence of chronic inflammatory mechanism and downregulation of antioxidant defense mechanism of cells are emerging major causes of neoplastic transformation and the progression of many solid cancers. The Longer the inflammation persists, higher the risk of developing many age related malignancies such as organs of colon, stomach and prostate etc. Concurrent occurrence of these processes that may affects DNA mutations in cells by excessive generation of free radicals, Reactive Oxygen Species (ROS) and other active intermediates that exceed the limit of the cells ability to neutralize it. Chronic inflammatory conditions may activate a variety of pro-tumorigenic activities, such as stimulation of proliferative pathway, chemotactic activity, increased invasive potential of cells and angiogenesis. However, many of the antioxidant or cellular redox molecules play a crucial role in maintaining cellular homeostasis and response to oxidative damage but down-regulation of some of the cellular molecules of this process may leads to malignant transformation.” – “Rel/NF-κB is a family of dimeric transcription factors that control the expression of numerous genes involved in the inflammatory process and other physiological responses of cells [57]. Many genes including TNFα, IL-1β, IL-6, IL-8, IL-12 cell adhesion molecules (vascular cell adhesion molecule-1 and intercellular cell adhesion molecule-1), iNOS, and COX-2 have been known to regulated via the NF-κb transcription factor [58-59]. Activation of many of these genes are involved in cell growth, differentiation, regulation of apoptosis, chemotaxis and metastasis progression in prostate and other solid cancers (Figure 2) [60-61]. These factors become activated by exposure to pro-inflammatory stimuli or activation of inflammatory responsive gene element that controls cellular redox balance mechanism [62-63], such as activation of oxygen-evoked sensitive HIF-1α and NF-κB. These two factors closely coupled with intracellular redox state or redox equilibrium and responsive to expression and trans-activation of many genes. So the differential regulation of HIF-1 α and NF-κB by oxygen-sensitive and redox-dependent pathways may play a role in inflammation related diseases [64] (Figure 3).”
Back to the central question: Is promotion of Nrf2 expression a viable strategy for human healthspan and lifespan extension?
I think the answer is probably YES based on the preponderance of research evidence as presented in these three blog entries. But it may be some time before this can be definitively proven.
Existing research such as cited in these three blog entries does establish:
- Increasing Nrf2 expression can help prevent, ameliorate or clear up numerous age-related health problems in a variety of species, problems ranging from Type 2 diabetes to ischemic stroke, to cancers to cardiovascular problems to recovery from physical injuries. To the extent diseases or conditions of infirmity can be prevented or cured people will live longer. “Longevity is the art of not dying.”
- Nrf2 expression is clearly associated with control of aging in a number of species. It is particularly high in certain long-lived species.
- Nrf2 expression can be practically increased by consuming a number of food substances, drugs and, particularly phyto-substance supplements such as those I have discussed in many other blog entries.
- Again, increasing expression of Nrf2 cannot be considered independently from acting on tightly linked pathways such as inhibition of expression of ND-kappaB and its inflammatory concomitants.
- If additional research establishes that induced expression of Nrf2 in humans leads to serious atherosclerosis or unacceptably raises plasma cholesterol levels and cholesterol content in the liver, then the YES answer I gave above might be switched to either NO or to researcher’s favorite fudbge answer which is “We don’t know yet. It will take more research to find out.”
So, the above is my answer to the question ““How can you simultaneously warn against indiscriminant antioxidant supplementation and at the same time so enthusiastically endorse consuming foods which have strong antioxidant capabilities, ones like broccoli, coffee, olive oil, chocolate, garlic, green tea and blueberries? And, if antioxidants are bad for you, how can you continue to advocate taking so many antioxidant supplements like curcumin, alpha-lipoic acid, ashwagandha, boswellia, ginger and resveratrol?” These substances primarily function via Nrf2 to promote the body’s own antioxidant and hormetic defenses and inhibit NF-kappaB and inflammation.” Their direct chemical antioxidant properties are of completely secondary importance.
Finally, I need emphasize that Nrf2 and NF-kappaB are only aspects of the ultimate conversation having to do with phytosubstances and aging. There are several important pathways that have to do with longevity and many of these are tightly linked. It is worth repeating some of the concluding observations from previous blog entries.
- Phytosubstances like curcumin, green tea, garlic and resveratrol are very complex and simultaneously affect multiple pathways. This makes the precise attribution of their health benefits a difficult task. For example, a large number of phytosubstances downregulate the expression of NF-kappaB (limiting the expression of inflammatory cytokines) and simultaneously promote the expression of Nrf2 (causing the expression of antioxidant and hormetic-protective genes). We often can identify a net positive health result but are unable to pinpoint the genes that cause them.
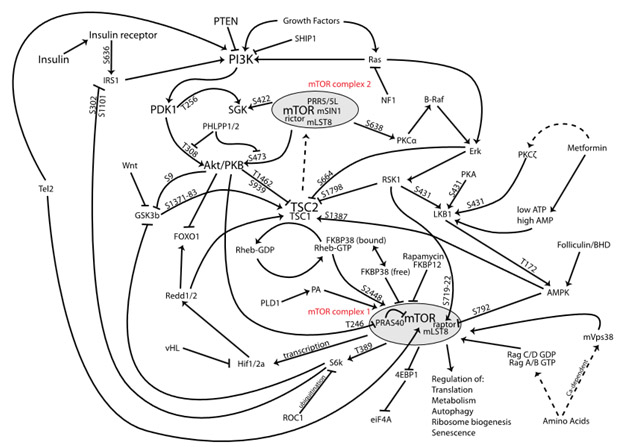
- Activation of pathways involved with metabolism and longevity such as Keap1-Nrf2, AMPK, PI3-kinase, AKT, mTOR, MAPK, PPAR-gamma, FoxO/DAF-16, the GH-IGF axis, P16(Ink4a), SIRT1, telomerase, Klotho and NF-kappaB seems to be more important in assessing the impacts of phytosubstances and their health-producing effects than activation of single genes.
- Dozens or hundreds of genes in pathways may be involved when something biologically important is going on, be that development of a cancer or Alzheimer’s disease or diabetes or be that a protective program that protects against such diseases.
- Complicated natural substances, phytochemicals, have evolved to address such pathways while traditional pharmaceuticals, simpler substances that address a single protein or gene or inhibit a single kinase, do not. That is one reason why pharmaceutical approaches to the age related diseases such as cancers, diabetes and Alzheimer’s disease have achieved only limited successes. And why the phytosubstances seem to offer so many possibilities
- Keap-1 – Nrf2 appears to be such a critical pathway. It appears that once the Keap1-Nrf2 pathway is activated it remains so for up to 24 hours, suggesting daily consumption of phytosubstances could be beneficial.
- New ways are coming to the fore for looking at health-producing effects of phytosubstances, such as epigenetic actions and effects of and on stem cells. These ways will yield new insights.
- There is much more to be written in the Antioxidant Chapter of the Great Book of Life before it is complete. But, a Chapter it remains.