By Vince Giuliano
This and the Part 1 blog entry Adaptogens Part 1 are pursuant to one of the key themes of this blog - the use of phyto-substances to promote health and longevity. The Adaptogens Part 1 blog entry discusses adaptogen herbs in general. This Part 2 blog entry is about a particular well studied adaptogen herb – rhodiola. Rhodiola is treateds from two viewpoints. First, the viewpoint of a practicing herbalist, Madelon Hope, is conveyed via a video presentation. Second, the viewpoint of current scientific research is covered in my usual manner: I cite a number of recent research citations relating to rhodiola involving its mechanisms of action and therapeutic potential. Most of my personal commentary regarding both adaptogens and rhodiola is in the concluding part of this blog entry.
The video features Madelon Hope, Director of the Boston School of Herbal Studies and a long-time practicing herbalist. I asked her for an informal interview which I recorded on a tiny video camera. The first part of the interview, on adaptogens in general, is included in theAdaptogens Part 1 blog entry. The segment of the video interview specific to rhodiola is included here.
MadelonHopePt2 from VinceGiuliano on Vimeo.
Some basics on rhodiola
There are many important subspecies of rhodiola plants, but the best known for health and medicinal purposes is Rhodiola rosea. Other subspecies mentioned here include Rhodiola Sachalinensis, Rhodiola Crenulataand Rhodiola integrifolia. Some scholars lump several subspecies under rhodiola rosea, others may see subspecies such as Rhodiola integrifolia as quite separate(ref). To further complexify the matter, the chemical constituents in the plant may vary in different subspecies and vary further due to soil and climate conditions.
According to Wikipedia: “Rhodiola rosea (also Golden Root, Rose Root, Roseroot, Aaron’s Rod, Arctic root, King’s Crown, Lignum Rhodium, Orpin Rose) is a plant in the Crassulaceae family that grows in cold regions of the world. These include much of the Arctic, the mountains of Central Asia, the Rocky Mountains, and mountainous parts of Europe, such as the Alps, Pyrenees, Carpathian Mountains, Scandinavia, Iceland, Great Britain and Ireland. — Rhodiola rosea may be effective for improving mood and alleviating depression. Pilot studies on human subjects[2][3][4] showed that it improves physical and mental performance, and may reduce fatigue. — Rhodiola rosea’s effects are potentially mediated by changes in serotonin and dopamine levels due to monoamine oxidase inhibition and its influence on opioidpeptides such as beta-endorphin,[5] although these specific neurochemical mechanisms have not been clearly documented with scientific studies. — Rhodiola is included among a class of plant derivatives called adaptogens which differ from chemical stimulants, such as nicotine, and do not have the same physiological effects. — In Russia and Scandinavia, Rhodiola rosea has been used for centuries to cope with the cold Siberian climate and stressful life.[6][citation needed] Such effects were provided with evidence in laboratory models of stress using the nematodeC. elegans,[7] and in rats in which Rhodiola effectively prevented stress-induced changes in appetite, physical activity, weight gain and the estrus cycle.[8] — Rhodiola has been used in traditional Chinese medicine, where it is called hóng jǐng tiān (红景天). — Rhodiola rosea contains a variety of compounds that may contribute to its effects,[10] including the class of rosavins which include rosavin, rosarin, and rosin. Several studies have suggested that the most active components are likely to be rhodioloside and tyrosol,[11] with other components being inactive when administered alone, but showing synergistic effects when a fixed combination of rhodioloside, rosavin, rosarin and rosin was used.[12]”
Salidroside (Rhodioloside) is “a glucoside of tyrosol found in the plant Rhodiola rosea. It is thought to be one of the compounds responsible for the antidepressant and anxiolytic actions of this plant, along with rosavin.[1][2] Salidroside may be more active than rosavin,[3] even though many commercially marketed Rhodiola rosea extracts are standardised for rosavin content rather than salidroside(ref).”
Until relatively recently it was thought that salidroside was a relatively inactive ingredient in rhodiola rosea and other key subspecies, probably not responsible for their major health benefits. “Although rosavin, rosarin, rosin and salidroside (and sometimes p-tyrosol, rhodioniside, rhodiolin and rosiridin) are among suspected active ingredients of Rhodiola rosea, these compounds are mostly polyphenols for which no physiological effect in humans is proved to prevent or reduce risk of disease.[13] (ref).” Now that view appears to be reversed and much of the recent literature reviewed below is concerned with health benefits of salidroside.
Image may be NSFW.
Clik here to view.
Rhodiola rosea Image source
A number of research publications deal with the capabilities of rhodiola to ameliorate conditions of stress and fatigue. Almost all the publications are positive with respect to the capabilities of the substance to exercise positive benefits. From the viewpoint of Western pharmaceutical science, however, the studies may be biased and collectively are probably inconclusive. More careful studies are needed.
The May 2012 review publication Rhodiola rosea for physical and mental fatigue: a systematic review represents this conservative point of view. “BACKGROUND: Rhodiola rosea (R. rosea) is grown at high altitudes and northern latitudes. Due to its purported adaptogenic properties, it has been studied for its performance-enhancing capabilities in healthy populations and its therapeutic properties in a number of clinical populations. OBJECTIVE: To systematically review evidence of efficacy and safety of R.rosea for physical and mental fatigue. METHODS: Six electronic databases were searched to identify randomized controlled trials (RCTs) and controlled clinical trials (CCTs), evaluating efficacy and safety of R. rosea for physical and mental fatigue. Two reviewers independently screened the identified literature, extracted data and assessed risk of bias for included studies. RESULTS: Of 206 articles identified in the search, 11 met inclusion criteria for this review. Ten were described as RCTs and one as a CCT. Two of six trials examining physical fatigue in healthy populations report R.rosea to be effective as did three of five RCTs evaluating R. rosea for mental fatigue. All of the included studies exhibit either a high risk of bias or have reporting flaws that hinder assessment of their true validity (unclear risk of bias). CONCLUSION: Research regarding R.rosea efficacy is contradictory. While some evidence suggests that the herb may be helpful for enhancing physical performance and alleviating mental fatigue, methodological flaws limit accurate assessment of efficacy. A rigorously-designed well reported RCT that minimizes bias is needed to determine true efficacy of R.rosea for fatigue.”
Personally, looking at many of the specific research publications, I find this conclusion rather harsh. Using the same logic, one could conclude that there are no clearly documented health benefits of drinking water, and a large scale clinical trial would be needed to clearly establish that there are such health benefits. My readers are invited to read on below and draw their own conclusions.
Some more-recent rat model studies have looked at actions of rhodiola components, particularly salidroside, on basic biological processes which happen in aging such as as decline in immune function, decline in DNA repair effectiveness, and increase in senescent cells. The reported results appear to be interesting if not dramatic.
The February 2012 e-publication Rejuvenating activity of salidroside (SDS): dietary intake of SDS enhances the immune response of aged rats reports: “It is well known that immune response decreases with aging. Salidroside (SDS), an antioxidant component isolated from the traditional Chinese medicine roseroot Rhodiola rosea, has been demonstrated to possess potent anti-aging and health-promoting activities. However, the mechanism underlying these activities is poorly understood. In this study, we clearly demonstrated that (1) dietary intake of SDS induced a considerable increase in total T cells (CD3(+)) and T helper cells (CD4(+)) in aged (21 months old) Wistar male rats; (2) SDS supplementation significantly increased the DTH response, a T cell-mediated immune response, in aged rats; and (3) SDS supplementation remarkably promoted the production of total anti-KLH IgG, anti-KLH IgG(1), and anti-KLH IgG(2α) in aged rats without disturbing immune homeostasis. These indicate that SDS is able to counteract immunosenescence, thereby resulting in rejuvenation. Practically, SDS may be used to help the elderly to generate an improved response to vaccine with stronger humoral and cell-mediated immune responses.” If this finding could be shown to carry over to humans, I believe that would be very exciting.
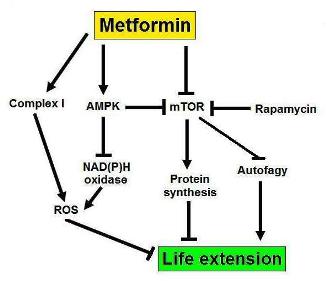
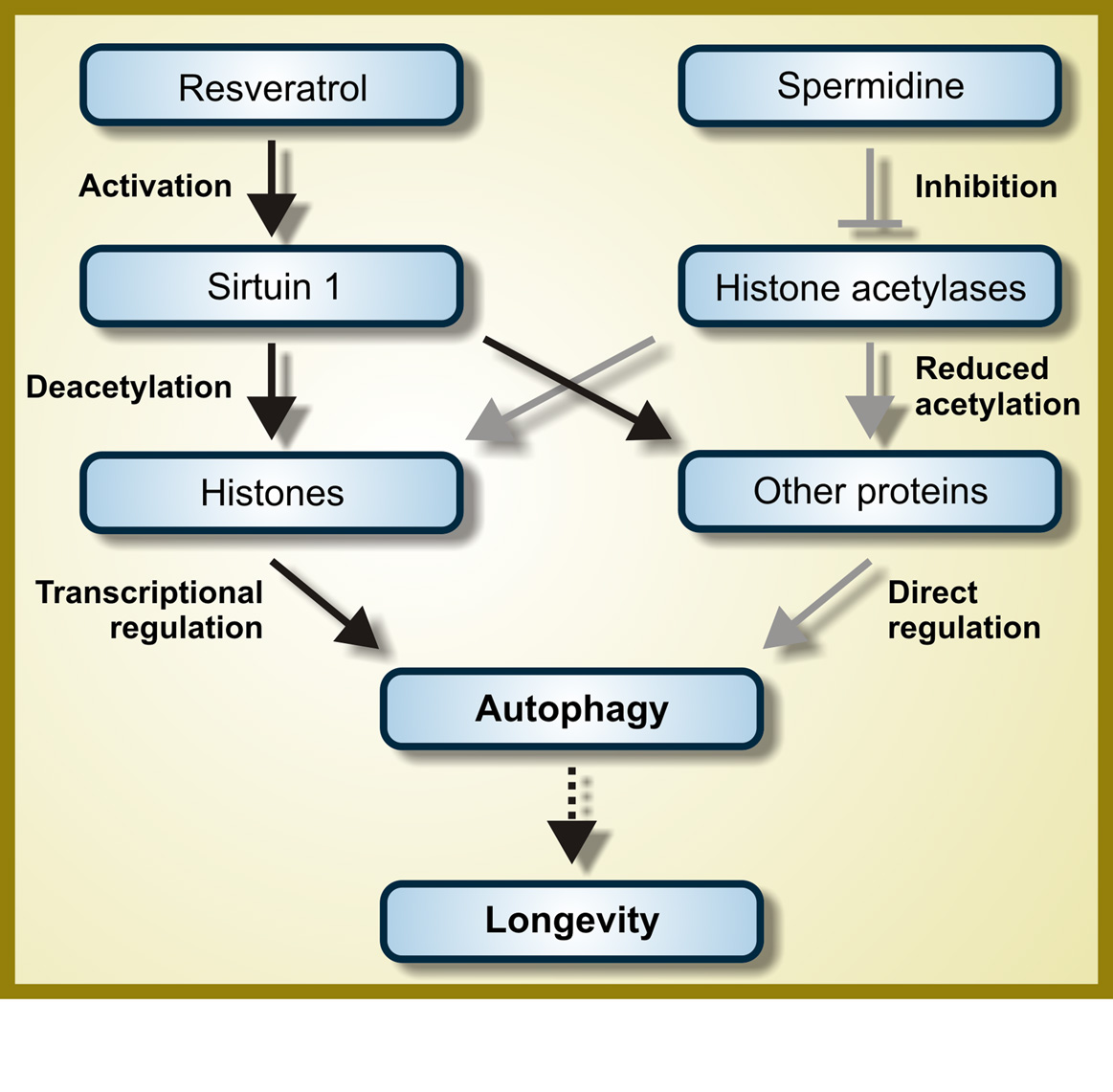
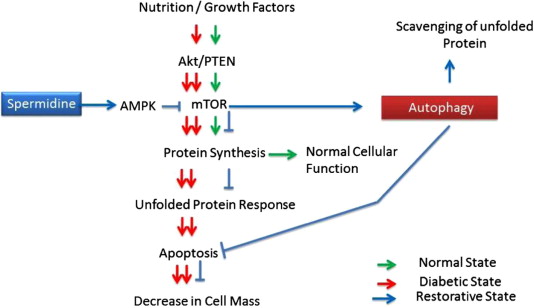
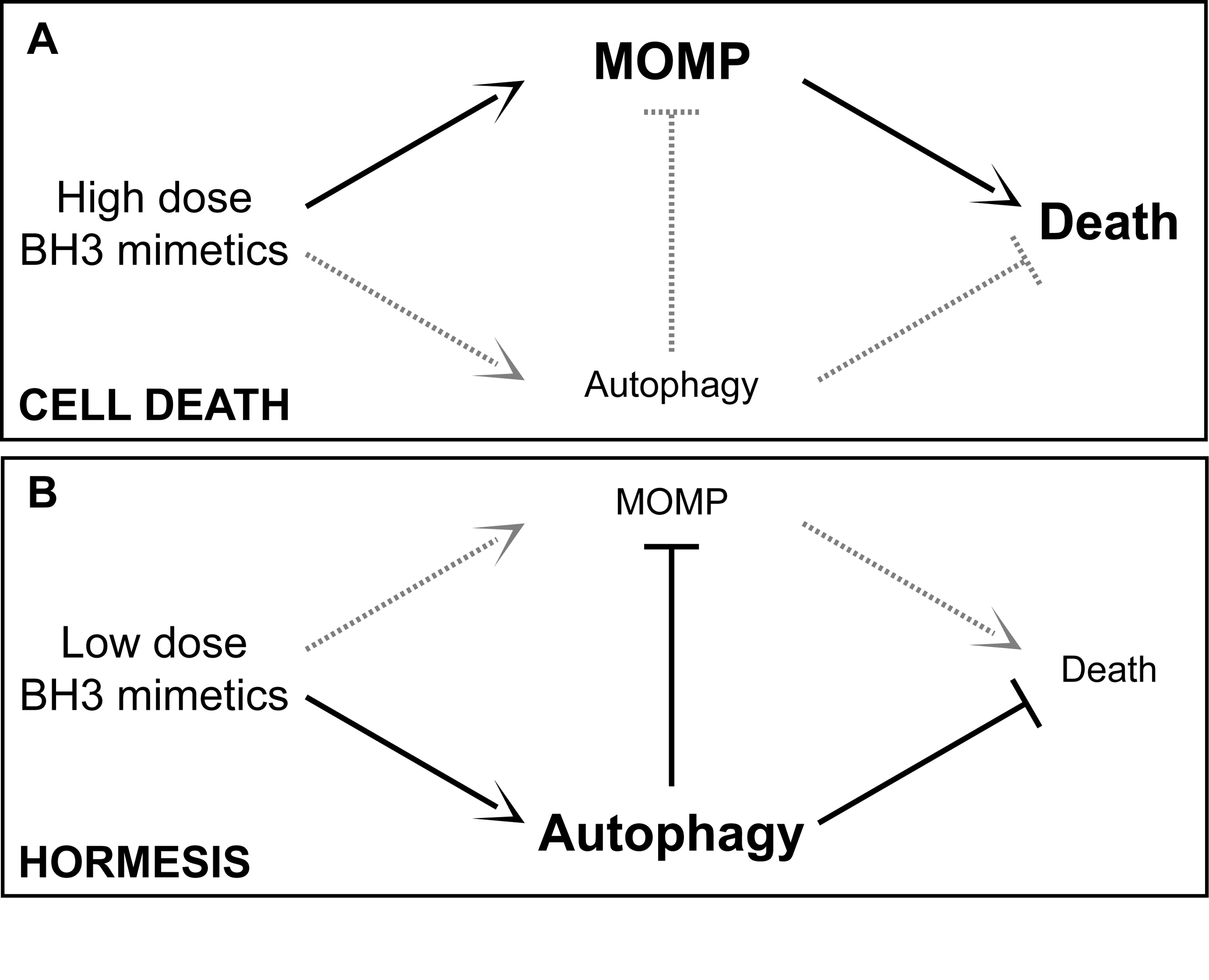
The May 2012 publication Salidroside stimulates DNA repair enzyme Parp-1 activity in mouse HSC maintenance also suggests a rejuvenating role of salidroside related to DNA repair and maintenance of hematopoietic stem cell pools. “Salidroside is a phenylpropanoid glycoside isolated from the medicinal plant Rhodiola rosea, which has potent antioxidant properties. Here we show that salidroside prevented the loss of hematopoietic stem cells (HSCs) in mice under oxidative stress. Quiescent HSCs were recruited into cell cycling on in vivo challenge with oxidative stress, which was blocked by salidroside. Surprisingly, salidroside does not prevent the production of reactive oxygen species but reduces hydrogen peroxide-induced DNA-strand breaks in bone marrow cells enriched for HSCs. We tested whether salidroside enhances oxidative DNA damage repair in mice deficient for 5 DNA repair pathways known to be involved in oxidative DNA damage repair; we found that salidroside activated poly(ADP-ribose)polymerase-1 (PARP-1), a component of the base excision repair pathway, in mouse bone marrow HSCs as well as primary fibroblasts and human lymphoblasts. PARP-1 activation by salidroside protects quiescent HSCs from oxidative stress-induced cycling in native animals and self-renewal defect in transplanted recipients, which was abrogated by genetic ablation or pharmacologic inhibition of PARP-1. Together, these findings suggest that activation of PARP-1 by salidroside could affect the homeostasis and function of HSCs and contribute to the antioxidant effects of salidroside.” As suggested in the previous Part 1 blog entry, I very strongly suspect that salidroside in part works by upregulating the expression of Nrf2 thus activating the body’s own natural antioxidant and repair defense systems.
The November 2010 publication Salidroside protects human fibroblast cells from premature senescence induced by H(2)O(2) partly through modulating oxidative status reports “Although salidroside and salidroside-like compounds are considered as most critical constitutes needed and responsible for multiple therapeutic benefits of Rhodiola rosea L., including anti-aging, direct demonstration regarding the role of salidroside in anti-aging process is still deficient. In this study, we selected the H(2)O(2)-induced premature senescence model in human fetal lung diploid fibroblasts to investigate the protection of salidroside against aging in vitro and associated molecular mechanisms. We found that salidroside considerably reversed senescence-like phenotypes in the oxidant challenged model, including alterations of morphology, cell cycle, SA-β-gal staining, DNA damage, as well as related molecules expression such as p53, p21 and p16. The protection occurred in a dose-dependent manner, with 5μM offering best efficacy. The proposed antioxidant property of the compound was confirmed in this cellular system, and thus at least partially accounted for the protection of the compound against premature senescence. Similar protection of salidroside against replicative senescence was observed as well. Interestingly, the regulation of senescence-related molecules by salidroside involved ROS-irrelevant mechanisms in both models. This finding presents salidroside as an attractive agent with potential to retard aging and attenuate age-related diseases in humans”
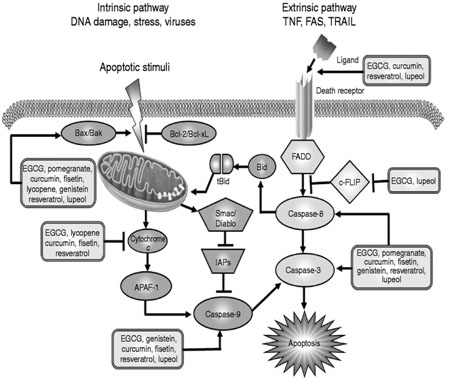
Multiple recent cell-level studies establish that salidroside definitely exercises cytoprotective effects, in part by inhibiting apoptosis in the presence of oxidative stress. Again. I suspect the effect is due to promotion of expression of Nrf2.
I cite only a few examples. For example, the April 2012 publication Salidroside protects human erythrocytes against hydrogen peroxide-induced apoptosis reports “Rhodiola rosea is a commonly used folk medicine for the treatment of high altitude sickness, mountain malhypoxia, and anoxia. Its active ingredient, salidroside [2-(4-hydroxyphenyl)ethyl β-D-glucopyranoside (1)], has been reported to have a broad spectrum of biological effects. However, the protective role of 1 in human erythrocytes remains unclear. This study therefore has investigated the effects of 1 on oxidative stress-induced apoptosis in human erythrocytes (also known as eryptosis or erythroptosis). Compound 1 increased cell survival significantly and prevented human erythrocytes from undergoing eryptosis/erythroptosis mediated by H(2)O(2), as confirmed by the decreased expression of phosphatidylserine on the cell surface and reduced leakage of calcein through the damaged membrane. Mechanistically, 1 was found to exert its protective effects through its antioxidative activity and the inhibition of caspase-3 activation and stress-induced intracellular Ca(2+) rise in a dose-dependent manner. Compound 1 is a protective agent in human erythrocytes against oxidative stress and may be a good adaptogen to enhance the body’s resistance to stress and fatigue.”
The July 2012 publication Salidroside and tyrosol from Rhodiola protect H9c2 cells from ischemia/reperfusion-induced apoptosis reports: “AIMS: Heart disease is the leading cause of death worldwide. Ischemia-reperfusion injury can lead to apoptotic death of heart cells and subsequently heart failure. Rhodiola is an herbal medicine with two main bioactive compounds – salidroside (SAL) and tyrosol (TYR). This study aimed to investigate whether these two compounds can prevent ischemia/reperfusion-induced apoptosis in H9c2 cells. MAIN METHODS: Assays for total phenolics assay and Oxygen Radical Absorbance Capacity showed high antioxidant capacity of SAL and TYR. H9c2 cells were subjected to simulated ischemia/reperfusion (IR) in the presence and absence of SAL and/or TYR, and nuclei condensation, caspase-3 activity, cytochrome c release and JNK phosphorylation were determined. KEY FINDINGS: In H9c2 cells, IR can lead to a 5-fold increase in p-JNK level. Apoptotic nuclei condensation, caspase-3 activity and cytochrome c release were markedly elevated, indicating the occurrence of apoptosis. SAL and TYR caused a dose-dependent inhibition of nuclear condensation. Furthermore, SAL and TYR, separately and in combination, significantly reduced caspase-3 activity, cytochrome c release and JNK activation. The anti-apoptotic effect of the combination was markedly higher than that of SAL or TYR alone. SIGNIFICANCE: The inhibition of the JNK signaling pathway is the key mechanism for the cytoprotective effect of SAL and TYR in IR-induced apoptosis.”
The August 2012 publication Protective effects of salidroside from Rhodiola rosea on LPS-induced acute lung injury in mice reports: “Salidroside is a major component extracted from Rhodiola rosea. In this study, we investigated protective effects of salidroside on lipopolysaccharide (LPS)-induced acute lung injury (ALI) in mice. In the mouse model, we found that pretreatment with a single 120 mg/kg dose of salidroside prior to the administration of intratracheal LPS induced a significant decrease in the W/D ratio and mouse myeloperoxidase activity of lung, reduction protein concentration, the number of total cells, neutrophils and macrophages in the bronchoalveolar lavage fluid. In addition, salidroside also inhibited the production of several inflammatory cytokines, including tumor necrosis factor-α, interleukin-6 (IL-6) and IL-1β, and the NF-κB DNA-binding activation after LPS challenge. These results indicated that salidroside possess a protective effect on LPS-induced ALI in mice.”
The 2011 publication Salidroside promotes erythropoiesis and protects erythroblasts against oxidative stress by up-regulating glutathione peroxidase and thioredoxin relates: “ETHNOPHARMACOLOGICAL RELEVANCE: Rhodiola rosea is commonly used in China and Tibet folk medicine for the treatment of high altitude sickness, anoxia and mountain malhypoxia. AIM OF STUDY: Salidroside (SDS) is an active ingredient of Rhodiola rosea. This study attempted to examine the potential erythropoiesis-stimulating and anti-oxidative effect of SDS in TF-1 erythroblasts. MATERIALS AND METHODS: The erythropoiesis-promoting effect was determined by treating human TF-1 cells, one of the popular in vitro models for studying erythropoiesis, with SDS in the presence and absence of erythropoietin (EPO) through the measurement of the expression of a series of erythroid markers such as glycophorin A (GPA), transferrin receptor (CD71) and hemoglobin (Hb). The potential protective effect of SDS against H(2)O(2)-induced apoptosis and its underlying mechanism in TF-1 erythroblasts were examined by flow cytometry and Western blot analysis. RESULTS: SDS promotes erythropoiesis in the EPO-treated cells and it also reduces the number of apoptotic cells in TF-1 erythroblasts after H(2)O(2) treatment probably through the up-regulation of protective proteins thioredoxin-1 (Trx1) and glutathione peroxidase-1 (GPx1). CONCLUSION: Our study provides evidence to explain the ethnopharmacological role of SDS and Rhodiola rosea in Chinese medicine. Our findings also support the use of SDS as an erythropoiesis-adjuvant agent to correct anemia and malhypoxia.” Again, we have here the familiar themes of protection against oxidative stress, prevention of apoptosis and stimulating stem cell differentiation.
The October 2011 publication Salidroside protects against hydrogen peroxide-induced injury in cardiac H9c2 cells via PI3K-Akt dependent pathway reports: “Oxidative stress induces serious tissue injury in cardiovascular diseases. Salidroside, with its strong antioxidative and cytoprotective actions, is of particular interest in the development of antioxidative therapies for oxidative injury in cardiac diseases. We examined the pharmacological effects of salidroside on H9c2 rat cardiomyoblast cells under conditions of oxidative stress induced by hydrogen peroxide (H2O2) challenge. Salidroside attenuated H2O2-impaired cell viability in a concentration-dependent manner, and effectively inhibited cellular malondialdehyde production, lethal sarcolemmal disruption, cell necrosis, and apoptosis induced by H2O2 insult. Salidroside significantly augmented Akt phosphorylation at Serine 473 in the absence or presence of H2O2 stimulation; wortmannin, a specific inhibitor of PI3K, abrogated salidroside protection. Salidroside increased the intracellular mRNA expression and activities of catalase and Mn-superoxide dismutases in a PI3K-dependent manner. Our results indicated that salidroside protected cardiomyocytes against oxidative injury through activating the PI3K/Akt pathway and increasing the expression and activities of endogenous PI3K dependent antioxidant enzymes.”
Another study relating salidroside to cardiac cells is reported in the 2009 publication Salidroside protects cardiomyocyte against hypoxia-induced death: a HIF-1alpha-activated and VEGF-mediated pathway. “Cardiomyocyte death (necrosis and apoptosis) plays a critical role in the progress of heart diseases. Salidroside, a phenylpropanoid glycoside isolated from Rhodiola rosea L, has shown cardioprotective effects in vivo. However, whether salidroside has a protective effect against cardiomyocyte death is poorly understood. The present study was aimed to investigate the cardioprotective role of salidroside and the underlying mechanisms in hypoxia-induced cardiomyocyte death. Cardiomyocytes pretreated with or without salidroside for 24 h were exposed to hypoxic condition for 6 h and then cell viability, necrosis, apoptosis, the expressions of HIF-1alpha and VEGF were investigated. Pretreatment with salidroside markedly attenuated hypoxia-induced cell viability loss, cell necrosis and apoptosis in a dose-dependent manner. Mechanistically, pretreatment with salidroside up-regulated the HIF-1alpha protein expression and induced its translocation. Moreover, the level of VEGF, a downstream target of HIF, was significantly increased in parallel with the level of HIF-1alpha following pretreatment with salidroside. However, 2-methoxyestradiol (2-ME2), a HIF-1alpha inhibitor, attenuated the protection of salidroside and blocked the increase of HIF-1alpha and VEGF. These data indicated that salidroside has protective effect against hypoxia-induced cardiomyocytes necrosis and apoptosis by increasing HIF-1alpha expression and subsequently up-regulating VEGF levels.”
A number of cell-level studies look at the protective effects of salidroside of neural cells and the ability of salidroside to promote neurogenesis under various in-vitro conditions and speculate whether it might become the basis for treatment of Parkinson’s disease, Alzheimer’s disease and other neurodegenerative disorders.
The January 2012 publication Protective effects of a Rhodiola crenulata extract and salidroside on hippocampal neurogenesis against streptozotocin-induced neural injury in the rat is a case in point. “Previously we have demonstrated that a Rhodiola crenulata extract (RCE), containing a potent antioxidant salidroside, promotes neurogenesis in the hippocampus of depressive rats. The current study was designed to further investigate the protective effect of the RCE on neurogenesis in a rat model of Alzheimer’s disease (AD) induced by an intracerebroventricular injection of streptozotocin (STZ), and to determine whether this neuroprotective effect is induced by the antioxidative activity of salidroside. Our results showed that pretreatment with the RCE significantly improved the impaired neurogenesis and simultaneously reduced the oxidative stress in the hippocampus of AD rats. In vitro studies revealed that (1) exposure of neural stem cells (NSCs) from the hippocampus to STZ strikingly increased intracellular reactive oxygen species (ROS) levels, induced cell death and perturbed cell proliferation and differentiation, (2) hydrogen peroxide induced similar cellular activities as STZ, (3) pre-incubation of STZ-treated NSCs with catalase, an antioxidant, suppressed all these cellular activities induced by STZ, and (4) likewise, pre-incubation of STZ-treated NSCs with salidroside, also an antioxidant, suppressed all these activities as catalase: reduction of ROS levels and NSC death with simultaneous increases in proliferation and differentiation. Our findings indicated that the RCE improved the impaired hippocampal neurogenesis in the rat model of AD through protecting NSCs by its main ingredient salidroside which scavenged intracellular ROS.”
The 2009 publication Protective effect of salidroside against H2O2-induced cell apoptosis in primary culture of rat hippocampal neurons relates : “Salidroside, a phenylpropanoid glycoside separated from a medicinal plant Rhodiola rosea, has been documented to have protective effects on neuronal cells in vitro. This study investigated whether salidroside was able to extend its unique neuroprotection to primary cultured rat hippocampal neurons against hydrogen peroxide (H(2)O(2))-induced cell damage. Cell viability tests and cell apoptosis assays confirmed that salidroside pretreatment attenuated H(2)O(2)-stimulated apoptotic cell death in primary culture of hippocampal neurons in a concentration-dependent manner. The measurements of caspase-3 activity, nitric oxide (NO) production, and NO synthase (NOS) activity suggest that the protection of salidroside, shown in this study, might be mediated by inhibiting caspase-3 activity, and antagonizing NO production and NOS activity during H(2)O(2) stimulation. Perhaps, this study might contribute to the development of salidroside as a broad-spectrum agent for preventing and/or treating neuronal damage in neurodegenerative disorders.”
The 2010 publication Neuroprotective effects of salidroside against beta-amyloid-induced oxidative stress in SH-SY5Y human neuroblastoma cells reported: “Beta-amyloid (Abeta) peptide, the hallmark of Alzheimer’s disease (AD), invokes a cascade of oxidative damages to neurons and eventually leads to neuronal death. In this study, salidroside (Sald), an active compound isolated from a traditional Chinese medicinal plant, Rhodiola rosea L., was investigated to assess its protective effects and the underlying mechanisms against Abeta-induced oxidative stress in SH-SY5Y human neuroblastoma cells. Abeta(25-35)-induced neuronal toxicity was characterized by the decrease of cell viability, the release of lactate dehydrogenase (LDH), morphological alterations, neuronal DNA condensation, and the cleavage of poly(ADP-ribose) polymerase (PARP) by activated caspase-3. Pretreatment with salidroside markedly attenuated Abeta(25-35)-induced loss of cell viability and apoptosis in a dose-dependent manner. The mechanisms of salidroside protected neurons from oxidative stress included the induction of antioxidant enzymes, thioredoxin (Trx), heme oxygenase-1 (HO-1), and peroxiredoxin-I (PrxI); the downregulation of pro-apoptotic protein Bax and the upregulation of anti-apoptotic protein Bcl-X(L). Furthermore, salidroside dose-dependently restored Abeta(25-35)-induced loss of mitochondrial membrane potential (MMP) as well as suppressed the elevation of intracellular reactive oxygen species (ROS) level. It was also observed that Abeta(25-35) stimulated the phosphorylation of mitogen-activated protein (MAP) kinases, including c-Jun NH(2)-terminal kinase (JNK) and p38 MAP kinase, but not extracellular signal-regulated kinase1/2 (ERK1/2). Salidroside inhibited Abeta(25-35)-induced phosphorylation of JNK and p38 MAP kinase, but not ERK1/2. These results suggest that salidroside has protective effects against Abeta(25-35)-induced oxidative stress, which might be a potential therapeutic agent for treating or preventing neurodegenerative diseases.”
Earlier publications on the effects of salidroside on neuroblastoma cells include the 2007 report Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells.
The neuroprotective effects of salidroside and the possibility of creating therapies for neurodegenerative diseases based on rhodiola compounds is far from new. For example there is a 2003 publication Salidroside from Rhodiola sachalinensis protects neuronal PC12 cells against cytotoxicity induced by amyloid-beta. “The amyloid beta-peptide (Abeta)-induced oxidative stress is a well-established pathway of neuronal cell death in Alzheimer’s disease (AD). Salidroside, one of the major compounds from the roots of Rhodiola species (Crassulaceae), was investigated in vitro for its cytoprotection against Abeta-induced toxicity on rat neuronal PCl2 cells. Salidroside significantly reduced Abeta-induced cytotoxicity in a dose-dependent manner. Salidroside also reduced Abeta-mediated intracellular accumulation of reactive oxygen species and malondialdehyde (MDA), a product of lipid peroxides, by preventing Abeta-induced decline of antioxidant enzyme activities. These results suggest that salidroside protects neuronal PC12 cells from Abeta-induced cytotoxicity via its antioxidant pathway.”
Fast-forwarding eight years to 2011, another report related to PC12 cells is Salidroside protects against MPP(+)-induced apoptosis in PC12 cells by inhibiting the NO pathway. “Oxidative stress plays an important role in Parkinson’s disease and other neurodegenerative disorders. Salidroside, a phenylpropanoid glycoside isolated from Rhodiola rosea L., has potent antioxidant properties. In the present study, we investigated the protective activity of salidroside against 1-methyl-4-phenylpyridinium (MPP(+))-induced apoptosis in PC12 cells. We found that incubation of PC12 cells with salidroside prior to MPP(+) exposure significantly reduced cell apoptosis and attenuated collapse of the mitochondrial membrane potential (MMP). Furthermore, salidroside inhibited the MPP(+)-induced nitric oxide (NO) increase and overexpression of nNOS and iNOS and suppressed accumulation of reactive oxygen species (ROS) and intracellular free Ca(2+). Our results show that the protective effects of salidroside on PC12 cells are mediated, at least in part, by inhibition of the NO pathway.”
Moving to right now, August 2012, we have the publication Salidroside protects PC12 cells from MPP(+)-induced apoptosis via activation of the PI3K/Akt pathway; “Oxidative stress plays an important role in the pathogenesis of Parkinson’s disease (PD). Salidroside (SAL), a phenylpropanoid glycoside isolated from Rhodiola rosea L., can exert potent antioxidant properties. In this study, we investigated the protective effects, and the possible mechanism of action, of SAL against 1-methyl-4-phenylpyridinium (MPP(+))-induced cell damage in rat adrenal pheochromocytoma PC12 cells. Pretreatment of PC12 cells with SAL significantly reduced the ability of MPP(+) to induce apoptosis in a dose and time-dependent manner. SAL significantly and dose-dependently inhibited MPP(+)-induced chromatin condensation and MPP(+)-induced release of lactate dehydrogenase by PC12 cells. SAL enhanced Akt phosphorylation in PC12 cells, and the protective effects of SAL against MPP(+)-induced apoptosis were abolished by LY294002, a specific inhibitor of phosphatidylinositol 3-kinase (PI3K) phosphorylation. These findings suggest that SAL prevents MPP(+)-induced apoptosis in PC12 cells, at least in part through activation of the PI3K/Akt pathway.”
Different species of rhodiola may induce differing biological effects.
The 2006 publication Evaluation of Rhodiola crenulata and Rhodiola rosea for management of type II diabetes and hypertension reported: “In the current study, we investigated 2 species of the genus Rhodiola for the inhibition of alpha-amylase,alpha-glucosidase and angiotensin converting enzyme (ACE) inhibitory activity. Water extracts of Rhodiola crenulata had the highest alpha-amylase inhibitory activity (IC50,98.1 microg total phenolic /ml) followed by ethanol extract of R.crenulata (IC50, 120.9 microg total phenolic/ml) and ethanol extract of R.rosea (IC50, 173.4 microg total phenolic /ml). Ethanol R.rosea (IC50, 44.7 microg total phenolic/ml), water extract of R.rosea (IC50, 52.3 microg total phenolic/ml), water extract of R.crenulata (IC50, 60.3 microg total phenolic /ml) and ethanol extract of R.crenulata (IC50, 60.2 microg total phenolic/ml) also showed significant alpha-glucosidase inhibitory activity. The alpha-glucosidase inhibitory activity of the extracts was compared to standard tyrosol, which was significantly detected in the extracts using HPLC. Tyrosol had strong alpha-glucosidase inhibitory activity (IC50, 70.8 microg total phenolic/ml) but did not have any inhibitory effect on the alpha-amylase activity. Results suggested that alpha-glucosidase inhibitory activities of both Rhodiola extracts correlated to the phenolic content, antioxidant activity and phenolic profile of the extracts. The ability of the above Rhodiola extracts to inhibit rabbit lung angiotensin I-converting enzyme (ACE) was investigated. The ethanol extracts of R.rosea had the highest ACE inhibitory activity (38.5 %) followed by water extract of R.rosea (36.2 %) and R.crenulata (15.4 %).”
Rhodiola may be useful for treatment of metabolic syndrome or Type II diabetes.
Pursuing a theme of the 2006 publication just mentioned , a new (August 2012) publication Rhodiola crenulata root ameliorates derangements of glucose and lipid metabolism in a rat model of the metabolic syndrome and type 2 diabetes aeports: “AIM OF THE STUDY: To examine the effects of Rhodiola on glucose and lipid metabolism in the metabolic syndrome and type 2 diabetes. MATERIALS AND METHODS: Zucker diabetic fatty (ZDF) rats were treated with Rhodiola crenulata root (RCR) powder (100 and 500mg/kg, by gavage, once daily for 4 weeks). In addition, the effects of RCR on sucrose-induced acute hyperglycemia in mice and olive oil-induced hypertriglyceridemia in rats were also examined. Biochemical variables were determined enzymatically or by ELISA. RESULTS: In ZDF rats, RCR treatment decreased the increased plasma insulin and triglyceride concentrations at baseline, the index of the homeostasis model assessment of insulin resistance (HOMA-IR) and excessive hepatic triglyceride accumulation. This treatment also inhibited abnormal increases in plasma glucose and insulin concentrations during oral glucose tolerance test. Furthermore, RCR reversed the increased adipose insulin resistance index, and accelerated the decline of plasma concentrations of non-esterified fatty acids after exogenous glucose stimulation. However, RCR minimally affected sucrose-induced acute hyperglycemia in mice and olive oil-induced acute hypertriglyceridemia in rats. CONCLUSIONS: The present results demonstrate that RCR treatment improves metabolic derangements in animal model of the metabolic syndrome and type 2 diabetes. Our findings may provide new pharmacological basis of therapeutics for the adaptogenic plants to treat metabolic derangements-associated disorders, such as asthenia.”
Some small clinical trials point to the safety and effectiveness of salidroside as a therapy or adjunct therapy for special disease conditions.
An example is discussed in the June 2012 publication Protective effects of salidroside on epirubicin-induced early left ventricular regional systolic dysfunction in patients with breast cancer. “Background: Salidroside [2-(4-hydroxyphenyl)ethyl-β-D-glucopyranoside], one of the most potent ingredients extracted from the plant Rhodiola rosea L., has been shown to have a cardiovascular protective effect as an antioxidant, and early treatment of epirubicin-induced cardiotoxicity has been the focus of clinical chemotherapy in patients with breast cancer. However, the cardioprotective effects of salidroside on epirubicin-induced cardiotoxicity, especially early left ventricular regional systolic dysfunction, have to date been sparsely investigated. Objective: The aim of this study was to investigate the protective effects of salidroside in preventing early left ventricular regional systolic dysfunction induced by epirubicin. Methods: Sixty patients with histologically confirmed breast cancer were enrolled. Eligible patients were randomized to receive salidroside (600 mg/day; n = 30) or placebo (n = 30) starting 1 week before chemotherapy. Patients were investigated by means of echocardiography and strain rate (SR) imaging. We also measured plasma concentrations of reactive oxygen species (ROS). All parameters were assessed at baseline and 7 days after each new epirubicin dose of 100 mg/m2. Results: A decline of the SR peak was observed at an epirubicin dose of 200 mg/m2, with no significant differences between salidroside and placebo (1.35 ± 0.36 vs 1.42 ± 0.49/second). At growing cumulative doses of epirubicin, the SR normalized only with salidroside, showing a significant difference in comparison with placebo at epirubicin doses of 300 mg/m2 (1.67 ± 0.43 vs 1.32 ± 0.53/second, p < 0.05) and 400 mg/m2 (1.68 ± 0.29 vs 1.40 ± 0.23/second, p < 0.05). Moreover, a significant increase in plasma concentrations of ROS was found with placebo, but they remained unchanged with salidroside. Conclusion: Salidroside can provide a protective effect on epirubicin-induced early left ventricular regional systolic dysfunction in patients with breast cancer.”
Small-scale human trials as well as animal studies suggests that salidroside can enhance endurance exercise performance.
The May 2012 publication The Effects of an Acute Dose of Rhodiola Rosea on Endurance Exercise Performance reports: “The purpose of this study was to determine the effects of an acute oral dose of 3mg/kg of Rhodiola Rosea (Rr) on endurance exercise performance, perceived exertion, mood, and cognitive function. Subjects (n=18) ingested either Rr or a carbohydrate placebo 1 h prior to testing in a double blind, random crossover manner. Exercise testing consisted of a standardized 10 min warm-up, followed by a 6-mile time trial (TT) on a bicycle ergometer. Perceived exertion (RPE) was measured every 5 min during the TT using a BORG 10 pt scale. Blood lactate concentration, salivary cortisol and salivary alpha amylase were measured pre warm-up, 2 min post warm-up, and 2 min post TT (n=15). A Profile of Mood States (POMS) questionnaire and a Stroop’s Color Test were completed pre-warm up and post TT. Testing was repeated 2-7d later with the other condition. Rr ingestion significantly decreased heart rate during the standardized warm up (Rr=136+17bpm; Placebo=140+17bpm; mean+SD; p=0.001). Subjects completed the TT significantly faster following Rr ingestion (Rr=25.4+2.7 min; placebo=25.8+3.0 min; p=0.037). The mean RPE was lower in the Rr trial (Rr= 6.0+0.9; placebo= 6.6+1.0; p=0.04). This difference was even more pronounced when a ratio of the RPE relative to the workload was calculated (Rr= 0.048+0.01; placebo= 0.057+0.02; p=0.007). No other statistically significant differences were observed. Acute Rr ingestion decreases heart rate response to sub-maximal exercise, and appears to improve endurance exercise performance by decreasing the perception of effort.”
Biological impacts of salidroside under exercise conditions are examined in a mouse model in the January 2012 publication [Influence of salidroside from Rhodiola Sachalinensis A. Bor on some related indexes of free radical and energy metabolism after exercise in mice]. “OBJECTIVE: To study the anti-fatigue mechanism of salidroside from Rhodiola SachalinensisA.Bor (SRS)in anti-oxidation and energy metabolic systems in mice , some related indexes of free radical and energy metabolism after exercise were measured. METHODS: Forty male mice were divided into four groups (n = 10): SRS sport group(SS), SRS quiet group(SQ),sport control group(SC), quiet control group (QC). The mice of SS and SQ groups received SRS solution of 150 ml/kg body weight per day for two weeks, while the mice of SC and QC groups received the same volume of distilled water. 30 min after the last treatment, the mice of SS and SC groups were forced to swim for 120 min without loads. then the biochemical parameters related to fatigue were determined. RESULT: SRS could increase liver superoxide dismutase (SOD) , glutathione peroxidase (GSH-Px) activity of antioxidant enzymes and reduce the malondialdehyde (MDA) content, which might increase the body activity of antioxidant enzymes to play the role of anti-oxidation; SRS had some effect of stabilizing blood sugar, increasing liver glycogen and muscle glycogen reserves, preventing blood sugar, liver glycogen and muscle glycogen levels from reducing in long time exercise on mice; SRS could increase plasma total cholesterol (TC), triglyceride (TG) and free fatty acid (FFA) levels in exercise mice, and it had some effect on metabolism of fat under different conditions, and promoted the use of the role of fat. CONCLUSION: Influence of SRS on some related indexes of free radical and energy metabolism is one of the mechanisms of anti-exercise-induced fatigue of SRS.”
A 2010 publication Effects of chronic Rhodiola Rosea supplementation on sport performance and antioxidant capacity in trained male: preliminary results relates: “AIM: Rhodiola Rosea, is an adaptogen plant which has been reported to promote fatty acids utilisation, to ameliorate antioxidant function, and to improve body resistance to physical strenuous efforts. The purpose of the present study was to investigate the effects on physical performance as well as on the redox status of a chronic Rhodiola Rosea supplementation in a group of competitive athletes during endurance exercise. METHODS: Following a chronic supplementation with Rhodiola Rosea for 4 weeks, 14 trained male athletes underwent a cardio-pulmonary exhaustion test and blood samples to evaluate their antioxidant status and other biochemical parameters. These data were compared with those coming from the same athletes after an intake of placebo. RESULTS: The evaluation of physical performance parameters showed that HR Max, Borg Scale level, VO(2) max and duration of the test were essentially unaffected by Rhodiola Rosea assumption. On the contrary, Rhodiola Rosea intake reduced, in a statistically significative manner, plasma free fatty acids levels. No effect on blood glucose was found. Blood antioxidant status and inflammatory parameters resulted unaffected by Rhodiola Rosea supplementation. Blood lactate and plasma creatine kinase levels were found significantly lower (P<0.05) in Rhodiola Rosea treated subjects when compared to the placebo treated group. CONCLUSION: Chronic Rhodiola Rosea supplementation is able to reduce both lactate levels and parameters of skeletal muscle damage after an exhaustive exercise session. Moreover this supplementation seems to ameliorate fatty acid consumption. Taken together those observation confirm that Rhodiola Rosea may increase the adaptogen ability to physical exercise.”
Also relevant to the exercise issue is the 2009 study Attenuation of long-term Rhodiola rosea supplementation on exhaustive swimming-evoked oxidative stress in the rat. “Rhodiola rosea improves exercise endurance and fatigue. We hypothesized that ingredients in Rhodiola rosea may increase antioxidant capability against swimming induced oxidative stress. In this study, we have identified the Rhodiola rosea ingredients, p-tyrosol, salidroside, rosin, rosavin and rosarin by high performance liquid chromatography-mass spectrometer and evaluated their O2(-)*, H2O2, and HOCl scavenging activities by a chemiluminescence analyzer. We next explored the effect and mechanism of Rhodiola rosea on 90-min swimming-induced oxidative stress in male Wistar rats fed with three doses of Rhodiola rosea extracts in drinking water (5, 25, 125 mg/day/rat) for 4 weeks. Our results showed that the 4 major ingredients (salidroside, rosin, rosavin and rosarin) from Rhodiola rosea extracts scavenged O2(-)*, H2O2, and HOCl activity in a dose-dependent manner. The ninety-min swimming exercise increased the O2(-)* production in the order: liver > skeletal muscle > blood, indicating that liver is the most sensitive target organ. The level of plasma malonedialdehyde, a lipid peroxidation product, was also increased after exercise. Treatment of 4 weeks of Rhodiola rosea extracts significantly inhibited swimming exercise-enhanced O2(-)* production in the blood, liver and skeletal muscle and plasma malonedialdehyde concentration. The expression in Mn-superoxide dismutase Cu/Zn-superoxide dismutase, and catalase in livers were all enhanced after 4 weeks of Rhodiola rosea supplementation especially at the dose of 125 mg/day/rat. Treatment of Rhodiola rosea extracts for 4 weeks significantly increased swimming performance. In conclusion, treatment of Rhodiola rosea extracts for 4 weeks could reduce swimming-enhanced oxidative stress possibly via the reactive oxygen species scavenging capability and the enhancement of the antioxidant defense mechanisms.”
Rhodiola might be useful for treating opioid addiction.
A May 2012 publication Effects of a Rhodiola rosea L. extract on the acquisition, expression, extinction, and reinstatement of morphine-induced conditioned place preference in mice reports: “RATIONALE: Opioid addiction is a chronic, recurrent brain disease that is characterised by compulsive drug seeking and a high rate of relapse even after long periods of abstinence. Prevention of relapse is the primary goal of addiction treatment and is still the major limitation in drug therapy. OBJECTIVES: The present study investigated the effects of a Rhodiola rosea L. hydroalcoholic extract (RHO), a well-known traditional oriental medicine, on establishment and reinstatement of morphine-induced conditioned place preference (CPP) in mice. METHODS: CPP was induced by intraperitoneal injection of morphine (10 mg/kg) as an 8-day conditioning schedule. The effects of RHO on the rewarding properties of morphine were tested in mice receiving oral administration of RHO (10, 15, and 20 mg/kg) 60 min prior to each morphine injection (acquisition) or prior to the CPP test on day 9 (expression). Once established, CPP was extinguished by repeated testing, during which conditioned mice were injected daily with different doses of RHO. Finally, the efficacy of RHO in blocking reinstatement of CPP provoked by priming injections and physical stress was also evaluated. RESULTS: RHO administration showed dose dependency for prevention of establishment of CPP and was effective in facilitating extinction of morphine-induced CPP. RHO suppressed both priming- and stress-induced reinstatement of CPP in a dose-dependent manner. CONCLUSIONS: In conclusion, as RHO was effective for reducing craving and vulnerability to relapse, it might be a very effective natural remedy for the treatment of opioid addiction.”
One of the traditional uses of rhodiola is for treatment of high-altitude mountain sickness. The benefits are probably due to the presence of salidroside
The March 2012 publication Salidroside stimulates the accumulation of HIF-1α protein resulted in the induction of EPO expression: a signaling via blocking the degradation pathway in kidney and liver cells reports: “Rhodiolae Crenulatae Radix et Rhizoma (Rhodiola), the root and rhizome of Rhodiola crenulata (Hook. f. et Thoms.) H. Ohba, has been used as a traditional Chinese medicine (TCM) to increase the body resistance to mountain sickness in preventing hypoxia; however, the functional ingredient responsible for this adaptogenic effect has not been revealed. Here, we have identified salidroside, a glycoside predominantly found in Rhodiola, is the chemical in providing such anti-hypoxia effect. Cultured human embryonic kidney fibroblast (HEK293T) and human hepatocellular carcinoma (HepG2) were used to reveal the mechanism of this hematopoietic function mediated by salidroside. The application of salidroside in cultures induced the expression of erythropoietin (EPO) mRNA from its transcription regulatory element hypoxia response element (HRE), located on EPO gene. The application of salidroside stimulated the accumulation of hypoxia-inducible factor-1α (HIF-1α) protein, but not HIF-2α protein: the salidroside-induced HIF-1α protein was via the reduction of HIF-1α degradation but not the mRNA induction. The increased HIF-1α could account for the activation of EPO gene. These results supported the notion that hematopoietic function of Rhodiola was triggered, at least partially, by salidroside.”
Comments
I could go on with many more citations and observations but think what is included above is sufficient to convey a good idea of what rhodiola’s capabilities are.
Collectively, these citations lead me to general observations applicable not only to salidroside and rhodiola but to most other traditional folk remedies: There have been more and more cell-level studies over the years establishing safety and efficacy of rhodiola and salidroside as an anti-oxidant and anti-apoptotic on the cell level. These cell-level studies are relatively easy and cheap to do and appear to me to be somewhat redundant over the years. Many of the newer studies are also unveiling the molecular pathways through which rhodiola and its important constituent compounds such as salidroside work. A smaller number of small-animal studies also establishing safety and efficacy for a number of health and medical conditions, and a yet-smaller collection of small-scale human trials have been held under various specialized conditions.
But, in the West at least, mainline salidroside-based clinical therapies that have been subject to pharma-scale clinical trials are yet to emerge. So salidroside and other traditional medical substances like andrographis, gambogic and gambogenic acids and icarin remain in the shadows. They are unknown to most physicians and unused in Western clinical practice. They may even be viewed as dangerous because of the possibility of their interactions with pharmaceutical drugs.
It is difficult to establish whether this is due to the weaknesses of the substances as drug candidates or due to the economics of clinical drug development in the Western model of medical practice involving pharmaceutical companies with a strong profit motive and the FDA as a watchdog. What drug company would want to spend a billion dollars and ten years to bring to market a natural substance that is not patentable, or an analog of such a natural substance that may be little or no better than the substance itself? And how many doctors are willing to risk administering substance therapies that are not FDA sanctioned?
So, in the US and other advanced Western countries, herbal healing remains a splinter activity unconnected with mainline medical practice. This may be slowly changing as 1. More medical schools and health institutions are starting programs in complementary, alternative or integrative medicine, 2. Big pharma and biotech companies turn more to traditional folk medicines in their searches for new drug candidates, and 3. There is increased public awareness among people that individuals need to take responsibility for their own health and longevity.
FROM TIME TO TIME, THIS BLOG DISCUSSES DISEASE PROCESSES. THE INTENTION OF THOSE DISCUSSIONS IS TO CONVEY CURRENT RESEARCH FINDINGS AND OPINIONS, NOT TO GIVE MEDICAL ADVICE. THE INFORMATION IN POSTS IN THIS BLOG IS NOT A SUBSTITUTE FOR A LICENSED PHYSICIAN’S MEDICAL ADVICE. IF ANY ADVICE, OPINIONS, OR INSTRUCTIONS HEREIN CONFLICT WITH THAT OF A TREATING LICENSED PHYSICIAN, DEFER TO THE OPINION OF THE PHYSICIAN. THIS INFORMATION IS INTENDED FOR PEOPLE IN GOOD HEALTH. IT IS THE READER’S RESPONSIBILITY TO KNOW HIS OR HER MEDICAL HISTORY AND ENSURE THAT ACTIONS OR SUPPLEMENTS HE OR SHE TAKES DO NOT CREATE AN ADVERSE REACTION.





















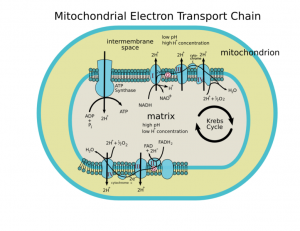
.svg/400px-Animal_mitochondrion_diagram_en_(edit).svg.png)



![Watson-2-150x150[1]](http://www.anti-agingfirewalls.com/__oneclick_uploads/2012/12/Watson-2-150x1501.jpg)